Car batteries are the unsung heroes of our vehicles. They are more than just simple boxes; they are sophisticated energy storage units that are indispensable for starting your car and powering its electrical systems. Whether you drive a traditional fuel engine car or a modern electric vehicle, a functioning battery is paramount. Upon ignition, your car battery unleashes stored chemical energy, converting it into the electrical power necessary to bring your vehicle to life. Let’s delve into the intricate world within that box and explore the essential Parts Of A Car Battery.
What Exactly is a Car Battery?
At its core, a car battery is a device engineered to convert chemical energy into electrical energy. Specifically designed for automobiles, these batteries store chemical energy and instantaneously transform it into electricity the moment you turn the key or press the start button.
The standard car battery is designed to be rechargeable and typically operates as a wet-cell battery. Regardless of the vehicle type, the fundamental role of all car batteries remains consistent: to reliably power the vehicle and all its accompanying electrical components.
When you initiate your car’s engine, the battery delivers a surge of electric current. This initial current is crucial; it ignites the internal combustion processes in fuel engines or activates the electric motors in EVs, setting your car in motion. Beyond starting the engine, the battery is also vital for powering a plethora of other electronic features, including headlights, infotainment systems, windshield wipers, and more, ensuring all these systems function efficiently.
Components That Power Your Ride: Inside a Car Battery
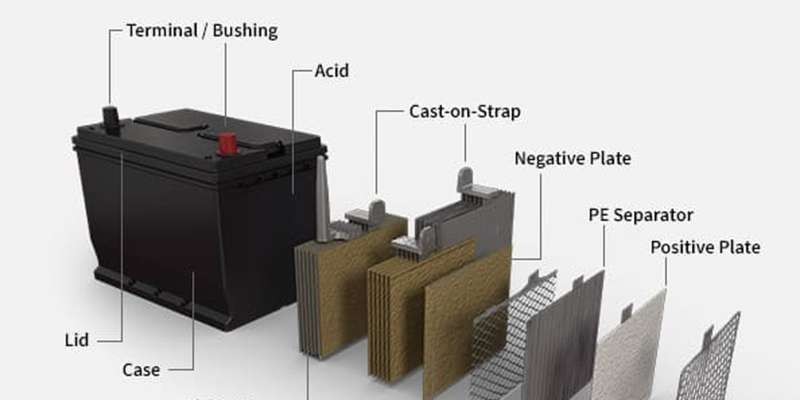
While a car battery may appear as a solitary unit encased in a box, its internal workings are a symphony of different components working in harmony. Let’s break down the essential parts of a car battery:
Battery Acid (Electrolyte)
Often referred to as the electrolyte, battery acid is a crucial component. In traditional lead-acid batteries, this is a solution meticulously formulated from sulfuric acid and water, typically ranging from 30 to 50 percent sulfuric acid concentration. This precise mixture acts as the electrolyte, the catalyst that sets off the necessary chemical reactions. These reactions are the foundation of electricity generation within the battery, ultimately powering your vehicle. The purity of this acid is paramount; contaminants can significantly impede its effectiveness in generating power for your car.
Upon ignition, the battery acid and the materials within the battery react, generating the required voltage to start your car. A weakened battery, with compromised acid or internal components, will struggle to produce sufficient voltage, leading to starting problems.
Battery Terminals
Battery terminals, sometimes called battery posts or bushings, are the vital connection points that bridge the car battery to your vehicle’s electrical system. Like any electrical circuit, a car battery features two distinct terminals: the positive (+) and negative (-) terminals. These are typically made of lead and are designed for secure and efficient connection with the car’s cables. The positive terminal is usually identified with a red color or a plus sign, and is slightly larger, while the negative terminal is black or marked with a minus sign and is slightly smaller.
Battery Case
Whether for a fuel-based or electric vehicle, the parts of the car battery are always housed within a protective enclosure known as the battery case. This case is critical for safeguarding the battery’s internal components from physical damage, vibrations, and environmental factors like temperature extremes and moisture. For conventional lead-acid batteries, the cases are often constructed from robust polypropylene resins, chosen for their durability and resistance to battery acid. In electric vehicles, where battery packs are often larger and more complex, the enclosure cases may be made from lightweight yet strong materials like aluminum alloys, which also aid in thermal management. The battery case is more than just a box; it’s a protective shield that significantly contributes to the battery’s longevity and reliable operation.
Battery Plates (Electrodes)
A typical car battery houses a series of positive and negative plates, also known as electrodes. These plates are the workhorses of the battery, where the electrochemical reactions occur. Each plate consists of a metallic grid, often made of a lead alloy. The positive plates are coated with lead dioxide, while the negative plates are made of spongy lead. These materials are chosen for their specific electrochemical properties that facilitate the flow of electrons and the generation of electrical current. Each plate has a cast-on strap at the top, which acts as a connector, linking each plate to the battery cells and forming a series of parallel circuits to increase current output.
Battery Separators
Battery separators are essential insulators positioned between the positive and negative plates within a car battery. These separators are thin, porous sheets, typically made of materials like polyethylene or other specialized plastic polymers. Their primary function is to prevent physical contact between the positive and negative plates, which would cause a short circuit, while still allowing the flow of ionic current through the electrolyte. Effective separators are crucial for maintaining the battery’s efficiency, preventing self-discharge, and ensuring reliable performance over its lifespan.
How Car Batteries Work: The Principle of Operation
The fundamental job of a car battery is to supply the electrical current needed to power all of a vehicle’s electrical systems. Even when your car is turned off, the battery continues to provide a low level of power to maintain essential functions like security systems and clock memory. However, the high-power demand for driving the car is only met when the engine is ignited.
Here’s a simplified look at the working principles of a car battery:
-
Chemical Reaction for Energy Conversion: When you turn the ignition, a chemical reaction is initiated within the battery. This process converts stored chemical energy into electrical energy. This electrical energy is then released to power the vehicle’s starter motor and provide initial voltage to the car’s electrical systems.
-
Voltage Stabilization: Once the car is started, the battery plays a crucial role in voltage regulation. It stabilizes the electrical current by ensuring a consistent voltage supply. Without this stabilization, unregulated voltage surges could develop, potentially damaging sensitive electronic components within your car.
It’s worth noting that starting a car engine typically requires only a small fraction of the battery’s total capacity, often around three percent. Car batteries are designed to deliver a high current burst for a short duration, primarily to start the engine and power essential ignition and lighting systems. This is why they are sometimes referred to as SLI batteries – Starting, Lighting, and Ignition – highlighting their primary functions.
A Look at Different Types of Car Batteries
The type of battery in your car can significantly influence its overall performance and reliability. Choosing the correct battery type for your vehicle is crucial for optimal operation. Let’s explore the different kinds of car batteries commonly available:
Primary Cell Batteries (Non-Rechargeable)
Primary cell batteries are designed for single use and are not rechargeable. Common examples include standard alkaline batteries like AA or AAA batteries, often used in household electronics such as remote controls. These batteries utilize materials like zinc and carbon. You will not typically find primary cell batteries in automobiles because of their inability to be recharged and their limited power capacity for automotive applications. They are also becoming less popular generally due to environmental concerns associated with disposal after a single use.
Secondary Cell Batteries (Rechargeable)
In contrast to primary cell batteries, secondary cell batteries are rechargeable, making them suitable for long-term and repeated use. They are composed of electrolytic materials, including an electrolyte and electrodes, which are essential for both energy storage and power delivery in vehicles. Secondary batteries are the standard choice for automobiles. The most common types include:
Lead-Acid Batteries
Lead-acid batteries are the traditional and most widely used type of rechargeable battery in vehicles, particularly in cars with fuel engines. These batteries were pivotal in the development of rechargeable battery technology and are known for providing a high power output relative to their size and cost. Beyond automotive applications, lead-acid batteries are also used in various industrial settings, such as hospitals and telecommunication towers, as reliable backup power sources due to their robust power delivery capabilities.
Lithium-Ion (Li-ion) Batteries
Lithium-ion batteries have become increasingly prevalent in the automotive industry, especially with the rise of electric vehicles (EVs). Li-ion batteries boast a high energy density, meaning they can store a significant amount of energy in a relatively lightweight package. This characteristic allows EVs to achieve longer driving ranges on a single charge. Additionally, Li-ion batteries exhibit a low self-discharge rate, which means they retain their charge effectively even when the vehicle is not in use for extended periods.
Solid-State Batteries
Solid-state batteries represent a cutting-edge advancement in battery technology. They are a relatively recent innovation aimed at overcoming the limitations of traditional liquid electrolyte batteries. The term “solid-state” refers to the use of a solid electrolyte material, typically a ceramic or glass-like substance, instead of the liquid electrolyte found in conventional batteries. This solid electrolyte makes solid-state batteries potentially safer, more energy-dense, and faster to charge. Although still in the early stages of widespread adoption, solid-state battery technology is rapidly gaining traction, particularly in the electric vehicle sector, due to its promising performance enhancements.
Key Functions of Car Batteries: More Than Just Starting the Engine
Car batteries perform several critical functions beyond just starting your engine. They are integral to the overall operation and reliability of your vehicle’s electrical system. Here are some of the primary functions:
Engine Starting Powerhouse
The most recognized function of a car battery is to provide the initial surge of power required to start the engine. Without a functioning battery, starting a car with an internal combustion engine is virtually impossible. The battery serves as the car’s primary power source at startup, converting stored chemical energy into electrical energy upon ignition. This electrical energy is then distributed to the starter motor and ignition system, bringing the engine to life.
Power Storage and Supply
A car battery acts as a reservoir of electrical energy, storing the power necessary not only for starting the car but also for running various electrical accessories. A well-maintained battery should retain enough charge to start the vehicle reliably, even after periods of storage. Once the engine is running, the alternator takes over the primary role of supplying power and simultaneously recharges the battery. This ensures that the battery remains charged and ready to provide power for subsequent engine starts and to supplement the alternator when electrical demands are high.
Collaboration with Alternator to Power Electrical Components
While the alternator is responsible for continuously powering the vehicle’s electrical systems while the engine is running, including lights, air conditioning, power windows, and the radio, the battery plays a crucial supporting role. The battery provides the necessary initial power to energize these systems and acts as a buffer, supplementing the alternator’s output when the electrical load exceeds the alternator’s capacity, particularly at low engine speeds or when many electrical accessories are in use simultaneously.
Voltage Regulation and System Protection
Modern car batteries are designed with features that help regulate voltage within the vehicle’s electrical system. Vehicle electrical systems can experience voltage fluctuations and surges, which can be harmful to sensitive electronic components. The battery acts as a stabilizer, absorbing excess voltage and preventing overvoltage conditions. This voltage regulation function helps protect the car’s electrical and electronic systems from damage, ensuring the longevity and reliability of these components.
Conclusion: The Unsung Hero Under the Hood
Car batteries are indispensable for vehicle operation. Understanding the parts of a car battery and their functions highlights their complexity and importance. From initiating the engine to stabilizing voltage and powering accessories, the battery is a critical component. Maintaining your car battery in optimal condition ensures reliable vehicle performance and prevents unexpected breakdowns. Regular checks and timely replacements when necessary are essential for every car owner to keep their vehicle running smoothly.
FAQs About Car Battery Components
What are the basic components of a car battery?
The fundamental components include the electrolyte (battery acid or lithium salt solution), positive plates (anode), negative plates (cathode), separators, terminals, and the battery case. The case is crucial for safety and containment of the battery’s internal components, preventing leaks and protecting users.
What is the liquid inside a car battery?
In traditional lead-acid car batteries, the liquid is the electrolyte, commonly referred to as battery acid. This is a solution of sulfuric acid and water. In lithium-ion batteries, the liquid electrolyte is a solution of lithium salts, such as LiPF6, LiClO4, or LiBF4, dissolved in organic solvents.