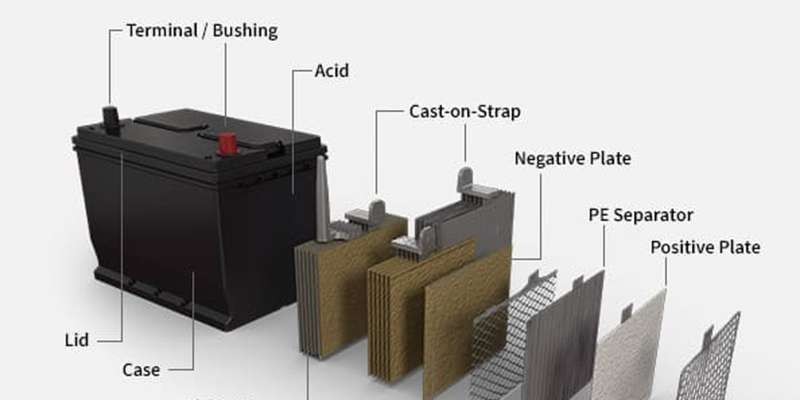
Car batteries are indispensable to the operation of any vehicle, acting as the primary energy storage unit. Whether your car is powered by a traditional fuel engine or an advanced electric motor, a functioning battery is crucial for ignition and overall performance. Far from being a simple container, a car battery is a sophisticated system designed to store and release energy precisely when needed to power your vehicle and its electrical systems.
In this guide, we will delve into the essential Car Batteries Parts, providing a detailed understanding of their functions and how they contribute to the overall operation of your vehicle.
What Constitutes a Car Battery?
At its core, a battery is a device engineered to convert chemical energy into electrical energy. A car battery, specifically, is designed to store chemical energy and rapidly convert it into electricity upon ignition. The vast majority of car batteries are rechargeable, employing a wet-cell design for efficient energy storage and delivery. Regardless of the vehicle type, the fundamental role of all car batteries remains consistent: to reliably power the vehicle and its diverse electrical components.
Upon starting your car, the battery immediately supplies a surge of electric current. This current initiates the internal combustion processes necessary for fuel-powered vehicles or activates the electric motors in EVs. Beyond starting the engine, the battery is also essential for powering a range of auxiliary electrical systems, including headlights, interior lighting, the radio, windshield wipers, and electronic control units. Without a properly functioning battery, these systems would be inoperable.
Components of a Typical Car Battery
Encased within a robust battery box or case, a typical car battery houses several critical internal car batteries parts. Understanding these components is key to appreciating how a battery functions. Let’s explore the essential parts:
Battery Acid (Electrolyte)
The battery acid, more accurately termed the electrolyte, is a solution composed of sulfuric acid and water, typically with a concentration ranging from 30 to 50 percent sulfuric acid in modern batteries. This carefully balanced mixture acts as the electrolyte, the medium that facilitates the chemical reactions necessary to produce the electric current. High purity is paramount for this solution, as any contaminants can negatively impact its effectiveness in generating power for the vehicle.
When the car is ignited, the electrolyte reacts with other internal components, generating the required voltage to start the engine. A weak or depleted battery will produce insufficient voltage, hindering the vehicle’s ability to start. In newer battery technologies, different electrolytes may be used, such as gelled electrolytes or absorbed glass mat (AGM) configurations to improve safety and reduce maintenance.
Battery Terminals
Battery terminals, sometimes referred to as battery bushings, are the crucial connection points that link the battery to the car’s electrical system. Like all electrical devices, a car battery features two terminals: a positive terminal (typically marked with a “+” sign and often larger in diameter) and a negative terminal (marked with a “-” sign). These terminals are usually made of lead or lead alloys for good conductivity and corrosion resistance. Clean and secure terminal connections are vital for ensuring efficient power transfer and preventing electrical issues.
Battery Case
The battery case is the external housing that encloses and protects all the internal car batteries parts. For traditional lead-acid batteries used in fuel-powered vehicles, the case is often constructed from durable polypropylene resins, chosen for their resistance to battery acid and physical impacts. In electric vehicles, where battery packs are significantly larger and more complex, the enclosure boxes are frequently made with lightweight yet strong materials like aluminum alloys. The primary function of the battery case is to safeguard the internal components from damage, vibration, and environmental factors, thereby contributing to the battery’s longevity and operational safety.
Battery Plates
Within each battery cell, there are sets of positive and negative plates. These plates are the sites of the electrochemical reactions that produce electrical energy. Each plate consists of a metallic grid, usually made of a lead alloy. The positive plates are coated with lead dioxide, while the negative plates are made of spongy lead. These materials are carefully chosen for their electrochemical properties and their ability to facilitate the reversible chemical reactions necessary for battery charging and discharging. Cast-on straps at the top of each plate assembly connect them to the battery cells and ultimately to the terminals.
Battery Separators
Battery separators are essential insulating layers positioned between the positive and negative plates within each battery cell. Their critical function is to prevent physical contact between the plates, which would cause a short circuit, while still allowing the flow of electrolyte ions. Separators are typically made from porous materials like polyethylene or other specialized plastic polymers. These materials must be resistant to battery acid and capable of maintaining their structure and insulating properties over the battery’s lifespan.
Diagram illustrating the various car batteries parts including terminals, case, plates, separator, and battery acid.
Working Principles of Car Batteries
The fundamental principle behind a car battery‘s operation is electrochemistry. When you turn the ignition key, a circuit is completed, allowing current to flow from the battery. This triggers a chemical reaction within the battery’s cells. In a lead-acid battery, for instance, sulfuric acid reacts with the lead dioxide on the positive plates and the spongy lead on the negative plates. This reaction generates electrons, which flow through the external circuit to power the starter motor and other electrical systems.
The primary role of the automobile battery is to supply the electrical current needed to power all of the vehicle’s electrical components. Even when the engine is off, the battery provides a small amount of power to maintain essential functions like the car’s computer memory and alarm system. However, the high current needed to start the engine is the battery’s most demanding task.
Here’s a breakdown of the working principles when you start your car:
-
Chemical Reaction Initiation: Upon ignition, a chemical reaction begins within the battery. This reaction converts stored chemical energy into electrical energy, providing the initial surge of power.
-
Voltage Stabilization: The battery plays a crucial role in voltage regulation. It ensures a steady flow of electric current by stabilizing the voltage. Without this regulation, uncontrolled voltage fluctuations could damage sensitive electronic components in the vehicle.
It’s important to note that starting a car typically requires only a small percentage of the battery’s total capacity, often around 3%. Car batteries are designed to deliver a high current for a short duration, primarily to start the engine and power the ignition system. This is why they are sometimes referred to as SLI batteries – Standing, Lighting, and Ignition – highlighting their primary functions. Once the engine is running, the alternator takes over the role of providing power to the vehicle’s electrical systems and recharging the battery.
Illustration depicting the working principle of a car battery in converting chemical energy to electrical energy to power a vehicle.
Different Types of Auto Batteries
The type of battery installed in your vehicle can significantly impact its performance and reliability. Selecting the correct battery type is essential for optimal operation. Here’s an overview of different types of vehicle batteries:
Primary Cell Batteries (Non-rechargeable)
Primary cell batteries are single-use, non-rechargeable batteries, such as common alkaline batteries (like AA or AAA batteries). These are not typically used in automobiles due to their inability to be recharged. While they were historically used in some very early automotive applications, they are not suitable for the high power demands and recharging requirements of modern vehicles.
Secondary Cell Batteries (Rechargeable)
Secondary cell batteries are rechargeable and designed for extended use cycles, making them the standard choice for automobiles. They consist of electrolytic materials (electrolyte and electrodes) that enable the reversible chemical reactions needed for charging and discharging. The most common types of secondary batteries used in cars include:
Lead-Acid Batteries
Lead-acid batteries are the most traditional and widely used type of car battery, particularly in vehicles with internal combustion engines. They were the pioneers of rechargeable battery technology and offer a good balance of power output and cost-effectiveness. Despite their relatively heavy weight and bulk, lead-acid batteries can deliver high current bursts needed for engine starting. They also find applications as backup power sources in settings like hospitals and telecommunication towers due to their robust power delivery capabilities.
Lithium-Ion (Li-ion) Batteries
Lithium-ion batteries have become increasingly prevalent in the automotive industry, especially with the rise of electric vehicles and hybrid cars. Li-ion batteries boast a high energy density, meaning they can store a significant amount of energy relative to their size and weight. This characteristic enables electric vehicles to achieve longer driving ranges on a single charge. Li-ion batteries also exhibit a low self-discharge rate, maintaining their charge for extended periods, even when the vehicle is not in use.
Lithium-Iron-Phosphate Batteries
Lithium-iron-phosphate (LiFePO4) batteries are a subtype of lithium-ion batteries that are gaining traction in automotive applications. They offer enhanced thermal stability and a longer cycle life compared to some other lithium-ion chemistries. LiFePO4 batteries are known for their safety and durability, making them a robust option for demanding automotive environments.
Image showcasing a traditional lead-acid car battery, highlighting its robust construction and terminal connections.
Solid-State Batteries
Solid-state battery technology represents a cutting-edge advancement in battery design. Unlike traditional batteries that use a liquid or gel electrolyte, solid-state batteries employ a solid electrolyte material, often a ceramic or glass-like substance. This innovation offers several potential advantages, including improved safety, higher energy density, and faster charging times. Solid-state batteries are emerging as a promising technology for electric vehicles, with the potential to significantly enhance EV performance and range.
Functions of Car Batteries
Beyond simply starting the engine, car batteries perform several crucial functions that are essential for the vehicle’s overall operation:
Engine Starter
The most critical function of a car battery is to start the engine. It acts as the vehicle’s power source, converting chemical energy into electrical energy to crank the engine and initiate the combustion process (in fuel vehicles) or activate the electric motors (in EVs). Without a functioning battery, starting the engine would be nearly impossible.
Power Storage
Car batteries store electrical energy, providing a reserve of power needed to restart the engine and operate various electrical accessories even when the engine is not running. A well-maintained battery should retain enough charge to reliably start the vehicle even after periods of storage. Once the engine is running, the alternator recharges the battery, ensuring it remains ready for subsequent starts and can continue to power electrical systems.
Collaboration with Alternator to Power Electrical Components
While the battery provides the initial power and starting current, the alternator takes over as the primary source of electrical power when the engine is running. The alternator generates electricity and supplies it to the vehicle’s electrical systems, including lights, air conditioning, power windows, and infotainment systems. The battery works in tandem with the alternator, providing supplemental power when needed and acting as a stabilizer in the electrical system.
Voltage Regulation
Modern car batteries are designed with features that help regulate voltage within the vehicle’s electrical system. In situations where other components might cause voltage surges or fluctuations, the battery can absorb excess energy and stabilize the voltage. This voltage regulation function protects sensitive electronic components from damage caused by overvoltage conditions, contributing to the overall reliability of the vehicle’s electrical system.
Get Car Battery Components at WayKen
The battery case is a vital component, safeguarding users and ensuring the integrity of all car batteries parts. For custom EV battery enclosure box design and manufacturing, WayKen offers expert solutions.
WayKen specializes in creating custom EV battery box designs tailored to your specific vehicle requirements. Beyond battery enclosures, WayKen provides comprehensive car part manufacturing services, including headlamps, backlights, and other automotive components.
Our team of experienced engineers and mechanics utilizes cutting-edge manufacturing technologies to fabricate high-quality car parts. We offer industry-leading CNC machining, injection molding, and 3D printing services. Simply upload your CAD files, and let WayKen bring your automotive component ideas to life. Visit our website to explore our capabilities in EV battery box design and car part manufacturing.
Conclusion
Maintaining your car battery in optimal condition is crucial for ensuring full access to your vehicle’s functionalities. If any car batteries parts are damaged or malfunctioning, it can compromise the battery’s performance and even render the vehicle inoperable. The battery case plays a vital role in protecting the internal components and ensuring user safety by containing potentially hazardous materials. Regular battery maintenance and timely replacement when needed are essential aspects of responsible vehicle ownership.
FAQs
What are the basic components of a car battery?
The fundamental car batteries parts include the electrolyte (such as sulfuric acid in lead-acid batteries or lithium salts in lithium-ion batteries), positive plates (anode), negative plates (cathode), separators, terminals, and the battery case. The case is crucial for containing the battery’s components safely and preventing leaks or accidents.
What is the liquid inside a car battery?
In a traditional lead-acid car battery, the liquid is the electrolyte, often referred to as battery acid because it is an acidic substance – a solution of sulfuric acid and water. In lithium-ion batteries, the electrolyte is a solution of lithium salts, such as LiPF6, LiClO4, or LiBF4, dissolved in organic solvents. These electrolytes facilitate the movement of ions necessary for battery operation.