Batteries are undeniably crucial for any vehicle. They serve as the power storage units that are essential for starting and running a car. Whether your vehicle is powered by a fuel engine or an electric motor, a functioning battery is indispensable for ignition and operation. Car batteries are more than just simple boxes; they are sophisticated energy reservoirs. Upon ignition, they release stored chemical energy, converting it into electrical energy to power your vehicle.
In this detailed guide, we will delve into the intricate parts of car batteries, explaining their functions and significance in the overall operation of your vehicle.
What is a Car Battery?
In simple terms, a battery is a device that converts chemical energy into electrical energy. A car or automobile battery is specifically designed to store chemical energy and rapidly convert it into electricity when needed, primarily upon starting the engine.
The standard car battery is designed as a rechargeable, wet-cell type. Regardless of the vehicle type, all car batteries perform the core functions of powering the vehicle and its various electrical systems.
When you turn the ignition key, the battery immediately supplies the electric current necessary to initiate the internal combustion process in fuel engines or power the electric motor in EVs. Furthermore, a wide array of your vehicle’s electronic components, including headlights, radio, windshield wipers, and more, rely on the battery for their operation.
Components of a Typical Car Battery
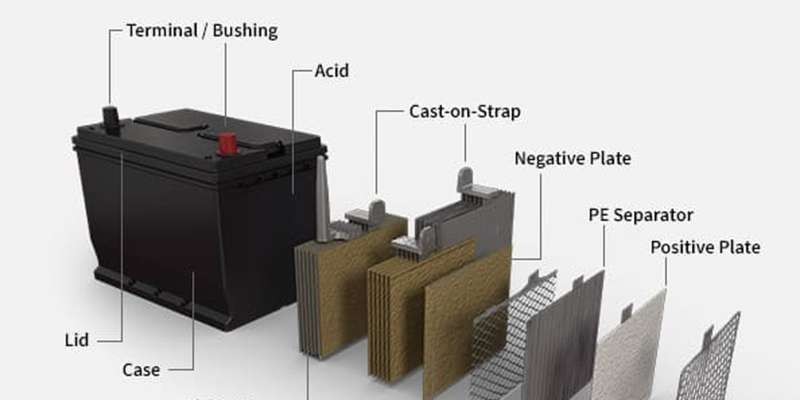
Although car batteries come in various shapes and sizes, a typical automobile battery is housed within a protective battery case or box. Inside this case, you’ll find a number of critical components working in concert. Let’s explore the essential parts of a car battery in detail:
Battery Acid (Electrolyte)
The battery acid, also known as the electrolyte, is a critical component. It is typically a solution composed of sulfuric acid and water, with the sulfuric acid concentration ranging from 30 to 50 percent in modern car batteries. This carefully formulated mixture acts as the electrolyte, facilitating the chemical reactions that generate the electric current necessary to power your vehicle. The purity of this acid is paramount, as contaminants can negatively impact its effectiveness in producing power.
When you start your car, the interaction between the battery acid and the battery plates triggers a chemical reaction that produces the required voltage to start the engine. A weak or depleted battery will struggle to produce sufficient voltage, potentially preventing the car from starting.
Battery Terminals
Battery terminals, sometimes referred to as battery bushings or posts, serve as the vital connection points between the car battery and the vehicle’s electrical system. Much like any electrical device, a car battery has two terminals: a positive terminal (+) and a negative terminal (-). These terminals are typically made of lead and are designed to securely connect to the car’s battery cables.
The positive terminal is usually slightly larger in diameter and often marked with a red color or a “+” symbol. Conversely, the negative terminal is slightly smaller and marked with black or a “-” symbol. These distinct markings are crucial to ensure correct battery connection, as reversed polarity can damage the vehicle’s electrical system.
Battery Case
The battery case is the robust outer shell that encases all the internal parts of the car battery. Whether in a fuel-powered car or an electric vehicle, the battery case plays a vital role in protecting the delicate internal components. For traditional lead-acid batteries used in fuel engines, the cases are often constructed from polypropylene, a durable and chemical-resistant plastic resin. In electric vehicles, where battery packs are often larger and more complex, the enclosures are frequently made from lightweight yet strong materials like aluminum alloys.
The primary function of the battery case is to provide physical protection to the internal components, shielding them from vibrations, impacts, extreme temperatures, and the harsh under-hood environment. This protection is essential for maintaining the battery’s integrity and prolonging its lifespan.
Battery Plates (Positive and Negative)
Inside every car battery are sets of positive and negative plates, which are fundamental to the battery’s electrochemical operation. Each plate consists of a metallic grid, typically made of a lead alloy for lead-acid batteries. However, the active material applied to these grids differs between positive and negative plates.
Positive plates are coated with lead dioxide (PbO2), while negative plates are coated with spongy or porous lead (Pb). These materials are specifically chosen for their electrochemical properties, allowing them to participate in the chemical reactions that generate electrical current. Each plate has a cast-on strap at the top, which connects it to the battery cells, creating a series of interconnected plates within each cell to increase the surface area for chemical reactions and thus the battery’s power output.
Battery Separator
The battery separator is a crucial, yet often unseen, component within a car battery. Its primary function is to electrically insulate the positive and negative plates from each other, preventing them from making direct contact and causing a short circuit. Despite preventing electrical contact, the separator must be porous enough to allow the flow of electrolyte between the plates, which is essential for the chemical reactions to occur.
Battery separators are typically made from porous, non-conductive materials such as polyethylene, fiberglass mats, or other specialized plastic polymers. The design and material of the separator are critical for battery performance and longevity, ensuring efficient ion transport and preventing internal shorts.
Working Principles of Car Batteries
The primary role of a car battery is to supply the electrical energy needed to power all of a vehicle’s electrical systems. Even when the engine is off, the battery provides a small amount of power to operate certain components like the car’s alarm system or internal lights. However, the battery’s most demanding task is to provide the surge of power required to start the engine.
Here’s a breakdown of the working principles of a car battery when you start your car:
- Chemical Reaction: When you turn the ignition key, a chemical reaction is initiated within the battery. This reaction converts the stored chemical energy into electrical energy. In lead-acid batteries, this involves a reaction between the sulfuric acid electrolyte and the lead plates.
- Voltage Supply: The battery delivers voltage to the car’s starter motor. This high-current discharge from the battery cranks the engine, initiating the combustion process in a gasoline engine or powering up the electric motor in an EV.
- Voltage Stabilization: Once the engine is running, the car’s charging system (alternator or generator) takes over the role of supplying power to the electrical systems and begins to recharge the battery. The battery also plays a role in stabilizing the voltage in the car’s electrical system, protecting sensitive electronics from voltage spikes and fluctuations.
It’s important to note that only a small percentage of a car battery’s total capacity is typically used to start the engine. Car batteries are designed to deliver a high current for a short duration—enough to start the engine and power the ignition and lighting systems. This is why they are often referred to as SLI batteries: Starting, Lighting, and Ignition.
Different Types of Car Batteries
The type of battery in your vehicle can significantly affect its performance and reliability. Choosing the right type of car battery is crucial for optimal vehicle operation. Here’s an overview of the different types of automotive batteries:
Lead-Acid Batteries
Lead-acid batteries are the most common type of car battery, and have been the industry standard for over a century. They are widely used in gasoline-powered vehicles due to their robust performance, reliability, and relatively low cost. Lead-acid batteries were instrumental in the development of rechargeable battery technology. They offer a good power-to-weight ratio and are capable of delivering high current bursts needed for engine starting. Beyond automotive applications, lead-acid batteries are also used in backup power systems, telecommunications, and industrial equipment.
Lithium-Ion (Li-ion) Batteries
Lithium-ion (Li-ion) batteries have become increasingly prevalent in the automotive sector, particularly with the rise of electric vehicles and hybrid cars. Li-ion batteries offer several advantages over lead-acid batteries, including higher energy density, lighter weight, and longer lifespan. They can store significantly more energy, enabling electric vehicles to achieve longer driving ranges. Li-ion batteries also exhibit a slow rate of self-discharge, meaning they retain their charge for longer periods, even when not in use. While initially more expensive than lead-acid batteries, the cost of Li-ion technology has been decreasing, making them a more attractive option for various automotive applications.
Solid-State Batteries
Solid-state batteries represent a cutting-edge advancement in battery technology. Unlike traditional batteries that use a liquid or gel electrolyte, solid-state batteries utilize a solid electrolyte material. This technology is still in its early stages of commercialization but holds immense potential for the future of automotive batteries. Solid-state batteries promise enhanced safety, higher energy density, faster charging times, and improved temperature stability compared to current lithium-ion batteries. They are expected to become increasingly important in electric vehicles, offering significant improvements in performance and range.
Functions of Car Batteries
Car batteries perform several vital functions beyond just starting the engine. They are integral to the overall electrical operation of a vehicle. Here’s a summary of the key functions:
Engine Starter
As previously mentioned, the primary function of the car battery is to provide the initial burst of energy required to start the engine. Without a functioning battery, starting a car with a conventional combustion engine would be virtually impossible. The battery acts as the powerhouse, converting stored chemical energy into electrical energy on demand, and delivering it to the starter motor.
Power Storage
The battery stores electrical energy, which is crucial for restarting the car and powering electrical accessories. A healthy battery should retain enough charge to start the vehicle even after periods of inactivity. Once the engine is running, the charging system (alternator) recharges the battery, ensuring it has sufficient power for subsequent starts and to support the vehicle’s electrical loads. The battery continuously cycles between discharging and recharging during vehicle operation.
Collaboration with Alternator to Power Electrical Components
While the battery provides the initial power, once the engine is running, the alternator becomes the primary source of electrical power for the vehicle’s systems. The alternator is responsible for powering components such as headlights, windshield wipers, air conditioning, radio, and various electronic control units. The battery works in conjunction with the alternator, providing supplementary power when electrical demand exceeds the alternator’s output, and also acting as a voltage stabilizer in the electrical system.
Voltage Regulation
Modern car batteries also contribute to voltage regulation within the vehicle’s electrical system. Fluctuations and surges in voltage can damage sensitive electronic components. Batteries can act as a buffer, absorbing excess voltage and helping to maintain a stable voltage level, thereby protecting the car’s electrical system from damage. This voltage regulation function is increasingly important in modern vehicles with complex electronic systems.
Conclusion
Car batteries are indispensable components in any vehicle, playing a crucial role in starting the engine and powering various electrical systems. Understanding the parts of a car battery, their functions, and the different types available can empower vehicle owners to make informed decisions about maintenance and replacements. Ensuring that your car battery is in optimal condition is essential for reliable vehicle operation and access to all of its functionalities. A properly maintained battery contributes to the overall performance, safety, and longevity of your vehicle.
FAQs
What are the basic components of a car battery?
The fundamental components of a car battery include the electrolyte (battery acid in lead-acid batteries or lithium salt solution in Li-ion batteries), positive and negative plates (anode and cathode), and the battery case. The battery case is critical for containing the battery’s components and preventing leaks or damage.
What is the liquid inside a car battery?
In a traditional lead-acid car battery, the liquid is the electrolyte, commonly referred to as battery acid. This is a solution of sulfuric acid and water. In lithium-ion batteries, the liquid electrolyte is a solution of lithium salts dissolved in organic solvents, such as LiPF6, LiClO4, or LiBF4.