Car batteries are essential for starting your vehicle and powering its electrical components. But have you ever wondered how your car battery stays charged while you drive? This guide will explain the component responsible for recharging your car battery and provide a comprehensive overview of your vehicle’s battery and charging system.
How Your Car Battery Initiates the Engine Start
The primary function of your car battery is to deliver the initial power needed to start the engine. Beyond this crucial role, it also stabilizes the electrical system, acting as a surge protector for the sensitive electronics and computer systems in your car. Additionally, it provides limited power to operate accessories like lights, radio, and wipers when the engine is not running.
The car battery is a key part of the starting system, which consists of three main components working in sequence:
- Ignition Switch: This is where you insert your key or press the start button. It’s the first point of contact to initiate the engine starting process.
- Starter Relay (Solenoid): When you turn the ignition, a small electrical signal is sent to the starter relay. This relay then acts like an electrical gate, closing contacts to allow a larger current to flow.
- Starter Motor: Once the starter relay closes, the battery sends a high-current jolt to the starter motor. This motor engages gears that physically turn the engine’s crankshaft, initiating the combustion process and starting the car.
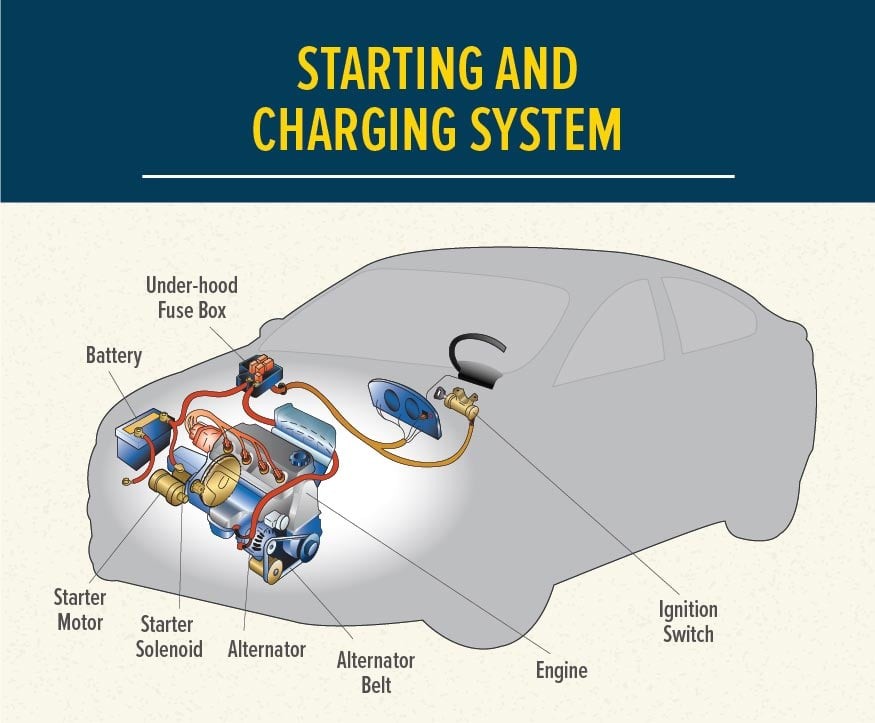 Vehicle Starting and Charging System
Vehicle Starting and Charging System
Alt text: Diagram of a vehicle starting and charging system, illustrating the flow of electricity from the battery to the starter motor and back to the battery via the alternator.
The Science Behind Car Battery Power
Alt text: Close-up photograph of a standard car battery with visible terminals and casing, highlighting the typical appearance of a lead-acid automotive battery.
Most car batteries, whether flooded or Absorbed Glass Mat (AGM), utilize lead-acid technology. Inside a typical lead-acid battery, you’ll find alternating plates of lead and lead compounds submerged in an electrolyte solution. This solution is a mixture of approximately one-third sulfuric acid and two-thirds water.
When you turn the ignition, a chemical reaction begins. The sulfuric acid in the electrolyte interacts with the active material on the battery plates. “Active material” refers to any substance within the battery that participates in the electrochemical reactions to either discharge or recharge the battery. This chemical reaction generates an electrical current, which is then channeled through the car’s starting system to crank the engine.
Decoding Cold Cranking Amps (CCA)
Cold Cranking Amps (CCA) is a critical rating for car batteries, especially in colder climates. CCA specifies the amount of current a battery can deliver for 30 seconds at 0 degrees Fahrenheit (-17.8 degrees Celsius) while maintaining a minimum voltage. Engines with larger displacements require more power to start, particularly in cold weather when engine oil thickens and components are stiff.
A higher CCA rating is beneficial, especially in regions experiencing freezing temperatures. In extremely cold conditions, a deeply discharged wet cell battery can freeze solid, potentially causing damage. Therefore, a robust CCA rating ensures reliable starting power even in harsh winter conditions.
The Alternator: The Heart of Your Car Battery Charging System
The alternator is the component in your car responsible for recharging the battery while the engine is running. Beyond recharging, the alternator also supplies the electrical power needed to operate your car’s electrical systems – such as headlights, infotainment, and power windows – when the engine is running.
The alternator is driven by a belt connected to the engine, commonly known as the serpentine belt or alternator belt. As the engine runs, this belt spins the alternator, generating electrical current. This generated current is then used to power the vehicle’s electrical components and simultaneously replenish the battery’s charge.
A voltage regulator plays a crucial role in managing the electrical flow from the alternator. It ensures a consistent and appropriate voltage level, preventing overcharging and delivering the correct amount of charge needed for various electrical demands, such as running the air conditioning or heater. The voltage regulator protects the battery from damage due to overcharging, which can shorten its lifespan.
Understanding Car Battery Degradation and Failure
Car batteries have a limited lifespan due to the repeated charge and discharge cycles they undergo. Each cycle causes minor wear and tear on the internal plates, leading to gradual deterioration of the lead components over time. As a car battery ages, its capacity diminishes, and consequently, the cold cranking amps (CCA) rating decreases.
Deep discharging is a significant factor contributing to premature battery failure. This occurs when the battery is heavily drained by using car accessories like the stereo or lights for extended periods while the engine is off. Repeatedly deep discharging a battery and then relying on the alternator to fully recharge it can cause sulfation. Sulfation is a process where sulfur from the electrolyte hardens on the lead plates, hindering the battery’s ability to accept and release charge, ultimately reducing its life.
Exploring Different Types of Car Batteries
Two primary types of car batteries are widely available today: standard wet cell batteries and absorbed glass mat (AGM) batteries. While both rely on lead-acid technology, they differ in construction and application.
Standard Wet Cell Batteries
Also known as flooded batteries, conventional batteries, or SLI (starting, lights, ignition) batteries, these are the most traditional type. Some wet cell batteries feature vents for releasing corrosive gases and condensation, often termed vented batteries. They may have removable caps for adding distilled water to maintain electrolyte levels. Conversely, some wet cell batteries are sealed systems without removable caps.
- Maintenance: Standard wet cell batteries require occasional maintenance, including cleaning corrosion from terminals and, for batteries with caps, topping off electrolyte levels with distilled water. Annual visual inspections are recommended, and battery charge levels should be checked before long trips and seasonally, particularly before temperature drops.
Absorbed Glass Mat (AGM) Batteries
AGM batteries represent a more advanced type, classified as valve-regulated lead-acid (VRLA) batteries. They are also known as sealed batteries, dry cell batteries, or non-spillable batteries. AGM batteries are sealed, preventing acid leakage and gas venting under normal operation. They incorporate pressure-activated relief valves that only open in extreme overcharging situations.
Modern vehicles, especially those with start-stop technology, often require AGM batteries. These batteries are designed to handle the increased electrical demands of such systems and consistently power vehicle electronics even when the engine is temporarily off.
AGM batteries offer advantages like longer charge retention, better tolerance to deep discharge cycles, and faster recharge rates compared to wet cell batteries. They also perform reliably in extreme temperatures. However, they are more susceptible to damage from overcharging.
- Maintenance: AGM batteries generally require less maintenance. Checking the charge level before long journeys and seasonal temperature changes is recommended.
It’s crucial to note that wet cell and AGM batteries are not interchangeable. Your vehicle is designed to operate with a specific battery type, so replacement should always be with the correct type specified by the manufacturer.